Abstract
INTRODUCTION: Acquired thrombotic thrombocytopenic purpura (TTP) is a rapidly fatal thrombotic microangiopathy mediated by autoantibodies targeting ADAMTS-13. The main pillars of acquired TTP management include therapeutic plasma exchange (PLEX) and immunosuppression with glucocorticoids and rituximab. PLEX is initiated in all patients with a presumptive clinical/confirmed diagnosis of TTP and is continued until platelet count recovery. The platelet response to PLEX is quite variable, with some patients having rapid recovery (early responders [ERs]) within 4-7 days, and others requiring a longer duration (late responders [LRs]) of PLEX. Although rapid platelet count recovery is reassuring to the treating physician, it is unclear whether there is a significant difference in outcomes between ERs and LRs to PLEX. We performed a single-center retrospective study of survival outcomes of ERs vs those of LRs with acquired TTP.
METHODS: We performed a retrospective analysis of adult patients (≥18 years old) with acquired TTP who were treated at our institution between January 1, 2005, and May 1, 2020. Baseline demographic and clinical data and laboratory values at the time of diagnosis were collected. Patients were ERs if platelet recovery (platelet count >150,000/µL for 2 consecutive days) occurred within 7 days of starting PLEX, whereas LRs had delayed platelet recovery (≥7 days) after initiation of PLEX. Refractory disease was defined as recurrent thrombocytopenia (platelet count <150,000/µL) following an initial response between days 1 and 30 after last PLEX. Relapses were defined as thrombocytopenia and elevated LDH occurring >30 days after last PLEX. We analyzed survival outcomes and the relapse risk in ERs versus LRs.
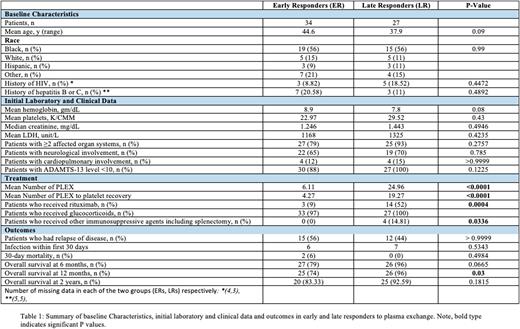
RESULTS: A total of 61 patients fulfilled the diagnostic criteria for acquired TTP (ER = 34 patients, LR = 27 patients). Patient demographics, laboratory studies, and treatment details are shown in Table 1. The mean age of ER and LR patients was 45 and 38 years, respectively. About 56% of patients in each group identified as Black. About 18% of ERs and 9% of LRs had a diagnosis of HIV. No statistically significant difference was noted in the baseline laboratory findings of the two groups. Involvement of ≥2 affected organ systems was seen in 79% of ERs and 93% of LRs (p=0.2757). All LRs (100%) and 88% of ERs had severe TTP at presentation (ADAMTS-13 level ≤10). The mean number of PLEX was 6.11 and 24.96 in ERs and LRs (p<0.0001), respectively. The mean number of PLEX to platelet recovery was 4.27 and 19.27 (p<0.0001). Glucocorticoids were given to 97% of ERs and 100% of LRs. First-line rituximab was given to 52% (n=14) of LRs, versus only 9% (n=3) of ERs (p=0.0004). About 15% of LRs went on to receive additional immunosuppressive agents, whereas no ERs did so (p=0.0336). The relapse rate among ERs and LRs was 55.56% and 44.44%, respectively (p > 0.9999). The 30-day mortality rate was 6% in ERs and 0% in LRs. Cardiovascular diseases were the common cause of early deaths. Overall survival (OS) at 6 months was 79% in ERs and 96% in LRs (p=0.0665). The OS at 1 year was 74% in ERs and 96% in LRs (p=0.03). The 2-year OS was available for 24 ER patients (83%) and LR 17 patients (89%) (p>0.9999). Among the 19 patients who received front-line rituximab, the OS at 1 year was 100%.
CONCLUSIONS: More LRs than ERs received rituximab and glucocorticoids for immunosuppression as first-line therapy. LRs were found to have a statistically significant greater OS at 1 year. ERs were less likely to receive adequate immunosuppression than LRs. This may indicate that the time to response to PLEX should not be used as a surrogate for risk of relapse. Immunosuppression is the cornerstone of the management of acquired TTP, and first-line use of rituximab is critical in the reducing relapse rate and improving OS. Larger studies in the prospective setting are needed to evaluate the impact of duration of PLEX on OS.
Disclosures
Idowu:Pfizer: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Ironwood: Research Funding; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Forma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal